British consumers could see lab-made meat and dairy on sale within two years, as the Food Standards Agency (FSA) is seeking to speed up the approval process.
A 'pioneering' regulatory programme has launched for cell-cultivated products (CCPs) - meat, dairy and sugar grown from cells in laboratories.
The FSA said it wanted to ensure these were safe for consumers before they’re sold, whilst supporting innovation in the sector.
As part of this, it has launched a two-year programme, alongside a team of scientists and regulatory experts, exploring the safety of CCPs for public consumption.
Their aim is to gather scientific evidence about CCPs and how they are made, to inform how the FSA would regulate these products.
In 2020, Singapore became the first country to authorise the sale of cell-cultivated meat for human consumption.
Since then, Italy and the US states of Alabama and Florida have instituted bans on CCPs in defence of traditional livestock farming.
But the UK's FSA said that lab-grown meat, dairy and sugar would deliver to consumers a 'wider choice of new food'.
"Safe innovation is at the heart of this programme," explained Professor Robin May, chief scientific advisor at the FSA.
"By prioritising consumer safety and making sure new foods, like CCPs are safe, we can support growth in innovative sectors."
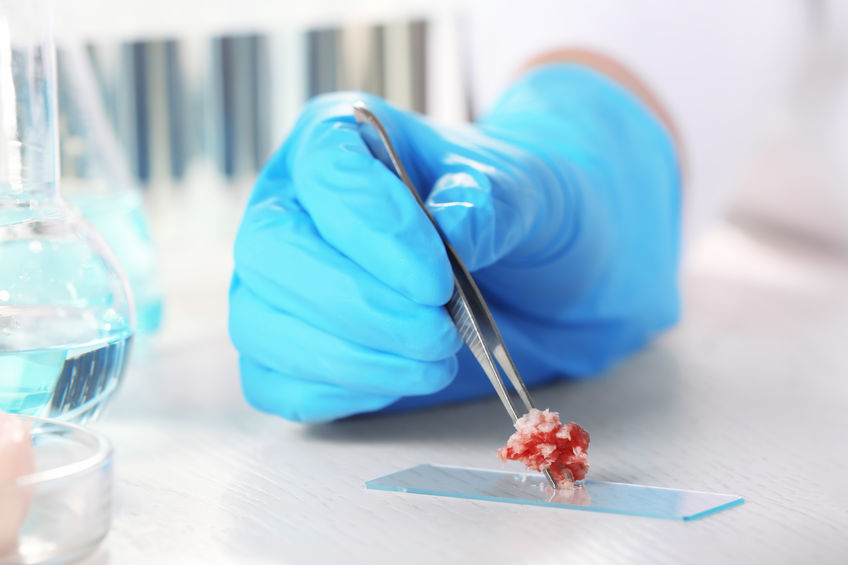
To create cultured or lab-grown meat, a biopsy is taken from an animal such as a pig or cow and then stem cells from that sample are placed in a bioreactor in a laboratory, where they are fed a solution of glucose, amino acids, vitamins and minerals.
This blend of cells and nutrients in specific conditions allows the cells to develop into mature muscle cells that forms cultured meat.
As part of the FSA's two-year programme, eight CCP companies have been selected to participate.
These include Hoxton Farms (UK), BlueNalu (US), Mosa Meat (The Netherlands), Gourmey (France), Roslin Technologies (UK), Uncommon Bio (UK), Vital Meat (France) and Vow (Australia).
