The Veterinary Medicines Directorate has issued a product defect recall for an antibiotic cream formulated for the treatment of bovine mastitis.
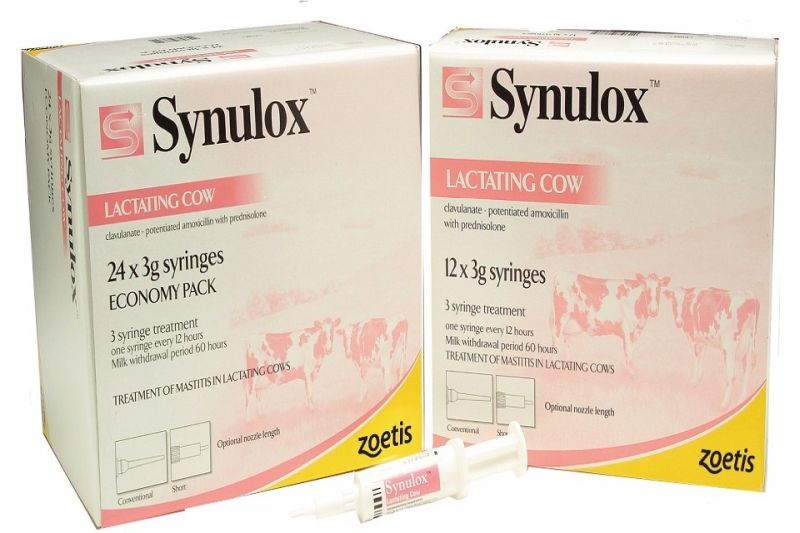
Zoetis UK's product - Synulox Lactating Cow Intramammary Suspension Vm 42058/4143 - has been recalled, the VMD announced in a statement.
It follows a recent Good Manufacturing Practice (GMP) inspection of one of the active pharmaceutical ingredient (API) manufacturers for Synulox.
As a precaution, batches of this product are being recalled from the market with immediate effect, the VMD said.
This issue impacts the following batches that have been placed on the UK market:
91939702 Synulox LC x 12 syringes 31/01/2021
81950600 Synulox LC x 24 syringes 30/04/2020
81958301 Synulox LC x 24 syringes 30/04/2020
81962601 Synulox LC x 24 syringes 31/05/2020
81966700 Synulox LC x 24 syringes 30/06/2020
81968102 Synulox LC x 24 syringes 30/06/2020
81968300 Synulox LC x 24 syringes 30/06/2020
91927402 Synulox LC x 24 syringes 30/11/2020
91938601 Synulox LC x 24 syringes 31/01/2021
91938700 Synulox LC x 24 syringes 31/01/2021
91954001 Synulox LC x 24 syringes 31/03/2021
Zoetis UK Ltd is contacting wholesale dealers and veterinary surgeons to examine inventory immediately and quarantine products subject to this recall.
The VMD's statement added that those concerned with the recall are told to contact Zoetis on 0845 300 8034.
